Carbon Dioxide Electron Arrangement
The multistep feature of CO 2 RR enables the production of various products determined by the used catalyst materials and operating. In this arrangement C 5-OH combines with the ketonic group present in the second position.

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
Electron affinity of Carbon is 1539 kJmol.

. Carbon dioxide CO 2 is another linear molecule consisting of two O-C bonds that are 180 degrees apart. It can be fatal if inhaled in large quantity. The first cAAC stabilized silylone was first reported by Mondal et al.
The change in energy in kJmole of a neutral atom or molecule in the gaseous phase when an electron is added to the atom to form a negative ion. In 2013 as a result of combusting fossil fuels with oxygen there was 390 ppm. Developing novel techniques to convert lignin into sustainable chemicals and functional materials is a critical route toward the high-value utilization of lignocellulosic biomass.
The CO 2 RR is a complicated reaction with several electronproton transfers and several articles have been published to propose different reaction mechanisms and pathways for several products. AX 2 - The two-electron domain structure produces a linear molecule with electron groups 180 degrees apart. This results in the formation of chiral carbon and two arrangements of CH 2 OH and OH group.
CO 2 was found in large concentrations for all test conditions reviewed in the literature as shown in Table 1The major component of first venting gas was CO 2 which was verified in experiments from literature 172628Overheating experiments for over 50 cells also indicated CO 2 as having the highest volume percentage in. These spare electrons in each carbon atom become delocalised. Remember that an elements electron cloud will become more stable by filling emptying or half-filling the shell.
Also shells dont stack neatly one on top of another so dont always assume an elements valence is determined by the number of electrons in its outer shell. In 2013 where cAAC is ligand used was CCH 2CMe 2 2 N-26-iPr 2 C 6 H 3. The complex was synthesized by reduction of cAAC 2 SiCl 2 a stable biradical precursor species with two equivalents of potassium graphite KC 8 reducing agent in tetrahydrofuran THF solution.
The animal has no need for the carbon dioxide and releases it into the atmosphere. Nitrogen dioxide is a chemical compound with the formula NO 2It is one of several nitrogen oxides. 150 years ago the natural concentration of carbon dioxide in the Earths atmosphere was 280 ppm.
A plant on the other hand uses the opposite reaction. AX 2 E and AX 2 E 2 - If. The bonding in graphite.
However an enhanced greenhouse. Carbon Dioxide CO 2. That leaves a fourth electron in the bonding level.
Nitrogen dioxide is a. In chemistry and atomic physics the electron affinity of an atom or molecule is defined as. NO 2 is an intermediate in the industrial synthesis of nitric acid millions of tons of which are produced each year for use primarily in the production of fertilizersAt higher temperatures it is a reddish-brown gas.
In this review the state-of-art sciences. This keeps the Earth warm enough to sustain life. Swiss and German researchers say this is the first known enzyme capable of directly using hydrogen as an electron source for carbon dioxide storage.
Each carbon atom uses three of its electrons to form simple bonds to its three close neighbours. Atmospheric carbon dioxide allows visible light in but prevents some infrared escaping the natural greenhouse effect. Hence D-fructose exhibits stereoisomerism in which α-D-fructopyranose and β-D-fructopyranose are the isomers.
Electronegativity of Carbon is 255. Here is a table of element valences. An example of a molecule with this geometry is CH 2 CCH 2 which has two H 2 C-C bonds forming a 180-degree angle.
2Mechanism of CO 2 RR and key challenges. Lignin-derived carbon materials hold great promise for applications in energy and chemical engineering catalysis and environmental remediation. In its metabolism of food and respiration an animal consumes glucose C 6 H 12 O 6 which combines with oxygen O 2 to produce carbon dioxide CO 2 water H 2 O and energy which is given off as heat.
This diagram is something of a simplification and shows the arrangement of atoms rather than the bonding.

Carbon Dioxide Molecule Co2 Lewis Dot Cross Electronic Diagram Covalent Bonds Ball Stick Space Filling 3d Models Boiling Point Melting Point Doc Brown S Chemistry Revision Notes

Co2 Molecular Geometry And Bond Angles Carbon Dioxide Youtube

Co2 Lewis Structure Easy Hard Science

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube
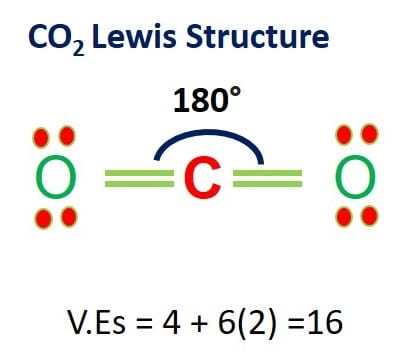
Co2 Lewis Structure And Molecular Geometry What S Insight

Learn About Chemistry S Octet Rule Electrons And Element Stability Persuasive Writing Prompts Chemistry Covalent Bonding Worksheet
0 Response to "Carbon Dioxide Electron Arrangement"
Post a Comment